 The four elements of traditional philosophy, soil, water, air, and fire, precisely correspond to the four states of the matter, solid, liquid, gas, and plasma (1).
The four elements of traditional philosophy, soil, water, air, and fire, precisely correspond to the four states of the matter, solid, liquid, gas, and plasma (1).
Let’s keep plasma aside for now. What distinguishes a solid body from liquid or gas? The answer: nothing, or rather nothing fundamental, besides appearance and consistency.
First of all, a few reminders.
Inside a molecule, the atoms are linked to each other by so-called covalent bonds. These bonds cannot be changed, except by denaturing the molecule, through a chemical reaction.
On the other hand, the molecules are linked to each other through so-called non-covalent bonds, also called extra-molecular bonds. They are of varied nature and can be changed through different methods. They are the ones that determine the state of matter:
-
Weak non-covalent bonds, meaning that the molecules are free in relation to each other = gas
-
stronger non-covalent bonds, movement of the molecules is limited = liquid.
-
Strong non-covalent bonds, numerous and structured, the movement of the molecules is very limited = solid.
It is the number and organization of covalent bonds that the hardness of solids is dependent on. But, pay attention: whatever its hardness, every solid can be deformed, it is a matter of time and temperature, both being linked.
As for temperature, it is obvious: we know well that a piece of red-hot iron becomes soft. High temperatures signify the augmentation of molecular agitation and consequently, the weakening of extra-molecular bonds.
As for time) it is less intuitive but just as obvious: what can occurs at high temperature happens just as well at room temperature, but rather slower. Therefore, the only difference between high and low temperature is the speed of the rearangment of extra-molecular bonds.
The most beautiful example of the time-temperature relationship is orogeny, i.e. the formation of mountains. In the Alps, it is enough for you to look around you to see the layers of geological folds that resemble marshmallows. The comparison is quite appropriate. Led by the internal movements, the earth’s surface is in perpetual motion. The continents are moving, colliding, and deforming. Over time, that is is millions of years, the rocks fall back, overlap, collide, and spread out, as true gummy. When earth forces are too strong, the energy cannot dissipate by deformation, but by dislocation, forming what geologists call faults.
Let us recall a fun science moment: The Ig Nobel prize, which “rewards” unusual or improbable research, has been awarded to researchers (several people, because one life is not enough) who observe a block of naphth, that is, solid tar. It forms a drop every . . . nine years. The experience lasted 80 years! (2). A slab of marble would behave similarly, but over thousands of years.
Water fits perfectly into this scheme, and stands out at the same time.
We know well that, in nature, water can be liquid state or gas (vapor). Moreover, it can be solid in the form of different types of ice – and not only in forms of ice. It is indispensable for living beings, to which they have adapted to meet the numerous challenges it poses.
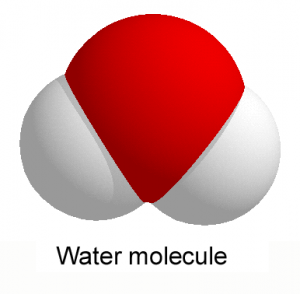
First of all, why is water water? The fact that it is a liquid assumes that the molecules are bound together by non-covalent bonds. This is easily explained by the structure of the molecule. It forms a triangle, in which the peak is occupied by oxygen (red in the figure), who has high electronegativity. In other words, an instant photo of the molecule would show the presence of more numerous electrons on the side of the oxygen atom, than those on the hydrogen’s side (colored gray). Although generally neutral, the molecule has a positive pole on one side and negative on the other; and is therefore polar. Consequently, between one molecule and another, the positive and negative poles attract each other, through electrostatic interactions, forming hydrogen bonds, the paragon, the model-type of non-covalent interactions.
Hydrogen bonds are essential formations of the living world, which they structure by establishing themselves inside proteins like other macromolecules, therefore stabilizing them as soon as they are formed.
Thus, water divides the physical world as well as the biological world into polar, hydrophilic, and other non-polar elements, hydrophobic (3). However, if water is, by definition, hydrophilic, it can also, paradoxically, be hydrophobic.
Otherwise, ALL GASES ARE HYDROPHOBIC, as Monsieur de la Palice says, they would be hydrophilic, so they would behave like water, therefore would not be gas. Another condition to fulfill, is small molecules, and how they are to be dispersed. Being non-polar and of large size, they would form fats or oils. Oxygen and nitrogen that make up the atmosphere meet these conditions, small-sized non-polar molecules. Conclusion, the atmosphere is a non-polar medium.
The water molecule is also of small size: its molar size is 18, in comparison with that of butane gas, 58 or 86 for hexane but, again, the non-covalent bonds limit the degree of freedom of molecules, which in effect form a liquid. Yet water also exists in the form of gas, it is water vapor. What happens in this case? Well, in water vapor, the molecules are completely dissociated from each other with homogenously-distributed electrostatic charges, eliminating polarity. Therefore, in vapor, water molecules are, paradoxically, non-polar.
As a liquid, water is, by definition, hydrophilic. In contrary, its vapor is hydrophobic.
This property is used (exploited?) by waterproof antiperspirant fabrics. They are provided with hydrophobic pores which act as a hydrophilic barrier to rainwater, but allow the hydrophobic passage of sweat and vapor from the sweat glands.
That is not all. The properties of water don’t end here.
It is known that snowflakes are formed of tiny ice crystals that are assembled into infinitely different structures, which makes each snowflake different from its neighbor. Millions and billions of different figures are built starting from a single unit, and ice crystal. The least of what we know is that this is “classic” ice, hexagonal ice. It is not the only possible ice form, because water molecules can organize differently to form another crystal, cubic ice (4).
Added to all the former are hydrated solids. All organisms, except animals and viruses, contain hygroscopic materials of a wide variety which constitute a significant fraction of the biomass. Although they are physiologically inert, they play an important role in nature by participating in the exchange of water between the environment and organisms, to which they provide mechanic properties of wide variety and complexity (5).
That is not all. The water of the living world does not cease to attract questions. So, how does sap, also known as water that is drawn from soil by the roots, rise to the top of the plants and large trees of more than tens of meters in height? There is no pump or treading or suction. In reality, there is indeed a sort of suction pump, it is evaporation. Arriving on the leaf, water evaporates through the stomata of leaves. Evaporation is controlled due to the microscopic pores (from 5 to 30 micrometers) which line the surface of leaves that open or close according to temperature conditions, lighting, and humidity. Therefore, it is a suction pump powered by solar energy. However, there are limitations to this mode of operation. The most efficient suction pump cannot aspirate a column of water for more than a few dozen meters in height. The plant’s answer: sap rises through capillaries in the wood’s vessels that consist of tubular-shaped cells stacked on top of each other, communicating through perforated walls. These perforations present with different structures among species, in response to different constraints that face the trees (6). These structures allow the plant to function in spite of diverse incidents that might perturb its mechanism, like cavitation (rupture of a sap column) or obstructions from air bubbles, or other obstacles.
- Other states of matter exist, that are rarely found in nature, but very important in our daily lives, like the amorphous state (glass), or liquid crystals, the ‘pope’ of which is the remarkable and regretted Pierre-Gilles de Gennes, winner of the Nobel prize in 1991. For more details, see Michael Mitov, Matière sensible (mousses, gels, cristaux liquides et autres miracles (Sensitive material, foams, gels, liquid crystals and other miracles), Editions du Seuil, 2010, ISBN : 978-2-02-095975-9.
-
IgNobels hail world’s longest-running experiment, 2005, Nature 437:938-9.
- Note that the term hydrophobic is incorrectly chosen, because it evokes a form of repulsion, while this is not the case. When these different products mix, the water molecules gather together, moving away from the hydrophobic elements, without there being any repulsion.
-
Huang X. et al., tracking cubic ice at molecular resolution, 2023, Nature617 (7959):86-91.
-
Harellson S. et al, Hydration solids, 2023, Nature 619 (7970) :500-5.
-
Structure of wooden vessels – Bing images


